Recovering from a Stroke with MSCs Stem Cells
Stroke is an abrupt interruption of constant blood flow to the brain that causes loss of neurological function, there are 3 types: a blocked artery (ischemic stroke) or leaking or bursting of a blood vessel (hemorrhagic stroke) and transient ischemic attack (TIA also known as a mini stroke ). Different factors can lead to a stroke: high blood pressure, high cholesterol, diabetes, and those who smoke. Mesenchymal Stem Cells (MSCs) are not only safe but also one of the only treatment that can improve the patient condition following a stroke. Study show that with Cerebral artery occlusion model the efficacy of interferon‐γ–activated mesenchymal stem cells (aMSCγ) as an acute therapy for stroke.
Hemorrhagic strokes account for 13% of all strokes, but are responsible for 40% of stroke-related deaths while ischemic stroke represent 87%. Moreover, up to 50% of stroke patients are still dependent on care 1 year after initial ictus and report impairments in memory, speech, and daily activities. Hemorrhagic stroke is caused by blood vessel rupture and subsequent extravasation of blood into the cranium, and can be further divided into subtypes based on the location of the bleed, including subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH), and intraventricular hemorrhage (IVH). Bleeding into the brain results in oxygen and glucose deprivation to perilesional tissue and initiates a secondary inflammatory response that contributes to lesion expansion, is detrimental to patient outcomes, and for which there is a dearth of therapeutics.
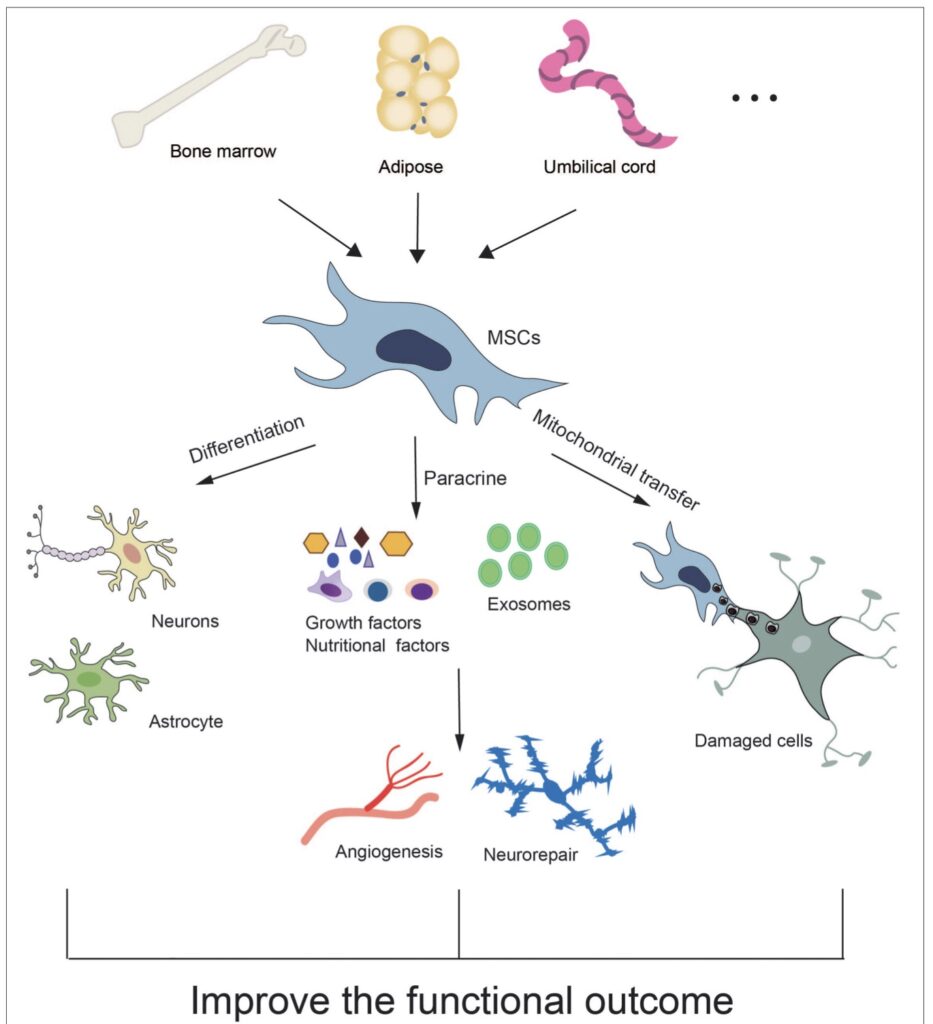
Studies used MSCs of human origin to treat intracranial hemorrhage. Around 60% of MSCs were sourced from bone marrow (BM-MSCs), as this is a viable source from both humans, whereas umbilical/placental/amniotic-derived cells were used in about one quarter of the studies, and the rest derived from adipose tissue (AT-MSCs). The latter sources were all derived from human tissue. MSCs were generally characterized by expression of cell surface markers assessed through flow cytometry or immunohistochemical methods. MSCs were positive for CD29, CD44, CD73, CD90, and CD105 among others, and negative for the hematopoietic lineage markers, CD14, CD34, and CD45, the stem cell marker CD133, and the marker for endothelial cells, CD144,30,31,32,33,34,35,36,37,38,39,40,41 which is consistent with guidelines.
Umbilical cord blood stem cells
Human umbilical cord blood stem cells (UC-BSCs) are derived from placental tissues, following birth. They consist of hematopoietic stem cells and MSCs. These cells offer a number of key advantages, such as an ample source of cells, low donor age, and low risks to babies and mothers during harvesting, which minimizes ethical concerns. These cells can differentiate into neural progenitor cells and provide neuroprotective effects in cerebral ischemia via neurotrophic factor secretion and vascular remodeling enhancement after stroke [62,63]. UC-BSCs have protective effects against ischemic injury, resulting in brainderived neurotrophic factor expression recovery [64]. In addition, UC-BSCs can inhibit the immune response and decrease the size of the ischemic brain lesion [65].
Stem cell administration routes
Intravenous delivery
IV delivery circumvents the need to access the CNS directly, thus allowing for less invasiveness [60,68]. Intravenously injected NSCs can traverse the BBB and localize to ischemic injury areas. NSCs, BM-MSCs, AD-MSCs, or UC-BSCs IV administration has shown neuroprotection in ischemic stroke [39,48,60,64,65,69].
Intra-arterial delivery
Intra-arterial (IA) delivery uses catheterization to guide stem cells into the carotid artery or circle of Willis, thus bypassing the initial uptake by systemic organs. IA administration has been shown to be advantageous for MSC delivery at the site of injury [78–80]. This delivery method allows for bypass of the lungs and avoids pulmonary entrapment [72]. While this method delivers a large number of cells to brain lesions, it also carries a risk of micro-embolism and cerebral ischemia due to arterial cell aggregation and clumping, resulting in reduced cerebral blood flow [81–83].Intraventricular delivery
Intraventricular delivery
Intraventricular delivery allows for direct delivery into the cerebral spinal fluid (CSF) or interstitial tissue, thereby allowing the dispersal to multiple sites of the brain and CNS through the CSF. Despite the invasiveness of this technique, complications can be reduced with the proper training and technique
Intraperitoneal
Intraperitoneal (IP) administration of human MSCs (hMSCs) led to the rapid aggregation of cells in the peritoneal cavity, and only a small amount of cells migrated elsewhere [89]. When compared to hMSC IV injection, IP administration resulted in a lower accumulation in peripheral tissues, such as the lungs and liver. However, IP-delivered umbilical cord-derived mesenchymal stromal cells (UC-MSCs) showed poor localization to the ischemic frontal cortex [90], suggesting limited brain penetration.
Intraparenchymal
Localized intraparenchymal delivery allows for a higher cellular volume to reach brain target lesions [91], thereby preventing offtargeting effects and improving cost-effectiveness. Intracranial transplantation of human AD-MSCs was shown to promote neuronal repair in cerebral ischemia-reperfusion injury [54]. MSC intracranial injection has been used for neonatal hypoxic-ischemic brain injury [92]. iPSC subdural injection upon infarcted brain tissue can reduce the infarct area size and improve motor function after ischemic stroke [93].
Intranasal delivery
Intranasal administration is a noninvasive and relatively effective drug or cell delivery route when compared to invasive intracranial transplantation. Although it has been recognized as a small agent (e.g., small molecules, proteins, viruses, bacteria, and nanoparticles) delivery method, its utility for cellular delivery has only been discovered in the last decade [98]. Intranasallydelivered cells collect beneath the nasal mucosa, close to the turbinate bones, and travel through the cribriform plate [99]. This method of delivery is non-invasive, rapid, and offers BBB bypass [100]. Cells showed improved targeting and less accumulation in peripheral organs, compared to systemic dosage [101]. Additionally, intranasal delivery allows for repeated dosages [102–105], making it more practical than other methods.

Comment (1)
interesting stuff, Lindas mum had a stroke, would be great to try and treat her but this covid situation doesnt make travelling very easy